Saponification
Saponification is the process that produces soap. The chemistry behind saponification appears complicated, especially if you look it up on Wikipedia. However, it's actually quite simple if you look at it from a different perspective.
If you think back to chemistry class, you'll remember the reaction between acids and bases. An example of an acid-base reaction is shown below:
HCl + NaOH → HOH + NaCl
HCl is Hydrochloric Acid. You've probably seen it sold at the hardware store as a concrete cleaner called muriatic acid. Hydrochloric acid is made of two atoms, hydrogen which is represented as H and chlorine which is represented as Cl. The part that makes hydrochloric acid an acid is that the positively charged molecule (cation, pronounced cat-ion) is hydrogen.
NaOH is Sodium Hydroxide. You've probably seen it sold at the hardware store as a type of drain cleaner. It comes in little white beads and will also be called lye. It's made of three atoms: sodium which is represented by Na, oxygen which is represented by O, and hydrogen which is represented by H. In bases like sodium hydroxide, the oxygen and hydrogen behave like a single atom called hydroxide which is represented as OH. What makes sodium hydroxide a base is that the negatively charged molecule (anion, pronounced an-ion) is hydroxide.
Acids and bases like to react with one another. This process is called neutralization. In this reaction, a strongly acidic and toxic hydrochloric acid molecule is neutralized by a strongly basic and toxic sodium hydroxide molecule and forms hydrogen hydroxide and sodium chloride.
The hydrogen from the hydrochloric acid has a strong affinity to react with the hydroxide from the sodium hydroxide to form the hydrogen hydroxide, represented by HOH. HOH can also be written as dihydrogen monoxide, represented by H2O. It's also known by its more common name, water.
The remainder of the acid, a chloride anion, and the remainder of the base, a sodium cation, have a strong affinity for one another and create sodium chloride. Generally, when non-hydrogen cations react with non-hyrdoxide anions, they form what is called a salt. In this particular case, the salt, sodium chloride, is table salt; the same table salt that you put on your food when you cook and eat.
The creation of soap is the same acid-base reaction, just more in-depth and with organic chemistry - the chemistry of carbon.
Organic chemistry is a very complex area of chemistry that would take much more than a hobbyist website to describe. Basically, its a series of molecules and reactions based upon long chains of carbon atoms which are also attached to extra hydrogen atoms.
An example of a carbon chain molecule is propane. Propane is C3H8. It looks like the image below.
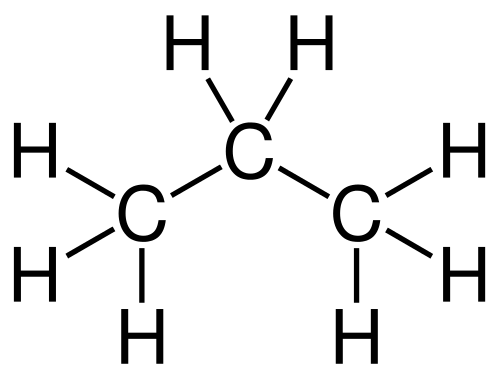
Organic molecules have acids and bases too. In fact, they're used far more often than inorganic acids and bases.
Organic acids have carbon chains, like propane, and then the carbon on the end of the chain replaces 2 of the attached hydrogens with a single, double bonded oxygen, and replaces the 3rd hydrogen with an oxygen atom which still has a vacant bonding site, so then a hydrogen is attached to it. An example is shown below.
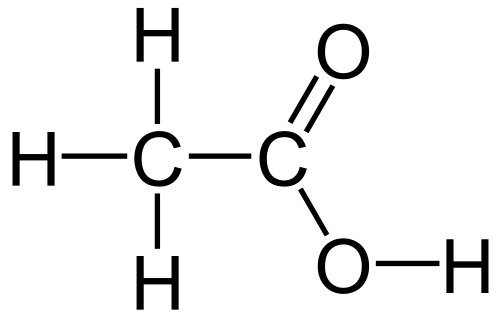
This particular acid has multiple names. If you note the two carbon atoms, it's based on ethane. That makes this acid ethanoic acid. The industrial name for this acid is acetic acid, and the commercial name is vinegar.
Organic bases are similar to organic acids. Using the same ethane base, the organic base is shown below.
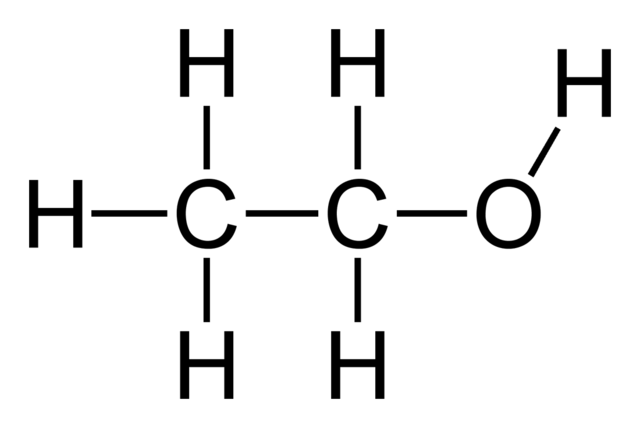
One of the carbons on the chain has had one of its hydrogen atoms replaced with an oxygen atom, and then that oxygen atom has a hydrogen atom attached to it. This compound is one of the most prolific compounds on Earth. Its chemical name is ethanol and it's sold commercially in a 40% ethanol, 60% water solution to anyone over the age of 21 in the United States, and it's called Vodka. The generic term for an organic base is an alcohol.
When organic acids react with organic bases, they form chemical compounds called esters, and water. Esters are the organic equivalent or salts. There are a variety of esters that have multiple properties. Some of them can be solvents like ethyl acetate which is formed by the reaction of vinegar and ethanol. Others are scents, like butyl butyrate (smells sweet, like fruit) which is formed by the reaction of butyric acid (C3H7COOH), the organic acid of butane (4-carbon-chain), and n-butanol (C4H9OH), the alcohol of butane where the hydroxyl group is on one of the end-carbons.
Soap is made from a series of esters that you know as fats. The technical term for the of large array of molecules commonly known as fats is a triglyceride.
Triglycerides start with an alcohol called glycerin.
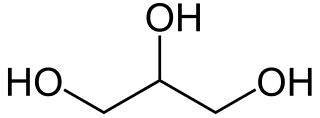
Glycerin is based on propane. The image above has removed the Cs for the carbons, but each intersection of lines represents a carbon, with each line representing a bond. There are 3 intersections, making 3 carbons. Then, at each carbon, there is a hydroxyl group attached. Glycerin is a thick, transparent liquid commonly found in many processed foods and in commercially available lotions. Glycerin, as an alcohol, is a weak base.
Then, a triglyceride takes 3 organic acids and neutralizes them at each of the hydroxyls on the glycerin. The theoretically simplest triglyceride is a glycerin molecule with three methanoic acid (formic acid, organic acid with only 1 carbon) molecules. However, this doesn't exist in nature. The vast majority of fats exist as oils like olive oil, coconut oil, and pork lard. They all have the same glycerin base, but they have extremely long carbon-chain acids attached (called fatty acids).
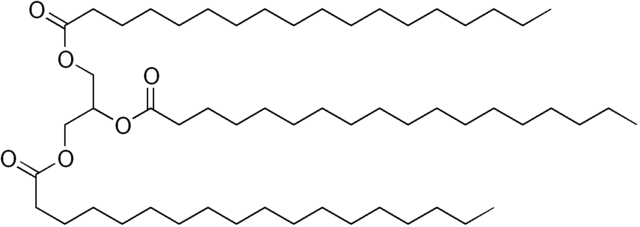
The image above is an example of a very specific type of triglyceride call stearin. It is three stearic acid molecules attached to a glycerin molecule.
Commercially available oils, Coconut oil, as an example, is not made of the same 3 acids attached to glycerin. It's a variety of acids. What makes it coconut oil is the average amount of each acid, which is shown in the table below.
| Name | Carbons | Formula | Amount |
|---|---|---|---|
| Caprylic | 8 | C7H15COOH | 7% |
| Capric | 10 | C9H19COOH | 8% |
| Lauric | 12 | C11H23COOH | 48% |
| Myristic | 14 | C13H27COOH | 16% |
| Palmitic | 16 | C15H31COOH | 9.5% |
| Oleic | 18 | C17H33COOH | 9.5% |
| Other | 5% | ||
The oleic fatty acid is the only acid on the list that is unsaturated. Unsaturated fatty acids have a double carbon bond that causes a bend to form in the shape of the acid.
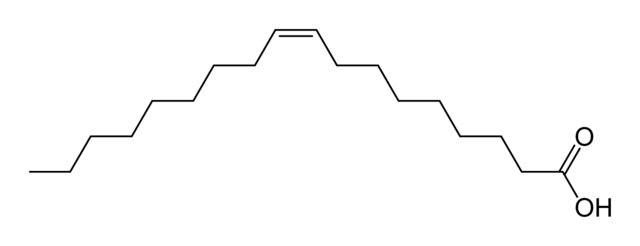
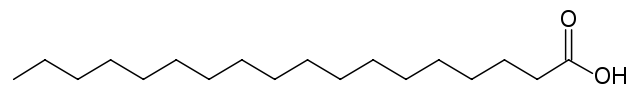
The images above are both 18-carbon-chain acids. The acid on the top is oleic. The acid on the bottom is stearic. Oleic acid has a double bond in the middle with a bend, making it an unsaturated fatty acid (omega-9 fatty acid, as the double bond is on the ninth carbon from the end). The bottom acid, stearic acid, has no double bonds, making it straight in shape. It is a saturated fatty acid. The majority of the acids (91%) in coconut oil are saturated fatty acids.
In general, the longer the carbon chains are, the more liquidy the oils tend to be at room temperature. Additionally, if the acids are unsaturated, they are more likely to be liquid at room temperature. Saturated fats and unsaturated fats have different properties when used to make soap.
Oils are unable to dissolve in water. That's why soap is needed to clean oily pots an pans. Soap acts as an emulsifier. It has the ability to dissolve both nonpolar organic molecules and polar inorganic molecules. Soap, as an emulsifier, has both a polar and nonpolar end of the molecule.
The actual saponification process turns an oil into into an emulsifier. One of the forms of lye, usually sodium hydroxide is dissolved in water and then that solution is added to oil. Because the sodium hydroxide is an extremely strong base, whereas the glycerin is a weak base, the sodium hydroxide bonds to the fatty acids and separates the glycerin. The sodium ion acts as the polarized end of the molecule, and the long carbon chain acts as the nonpolar end.
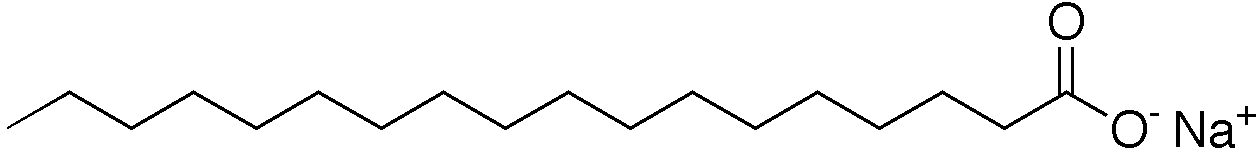
The image above shows sodium stearate, one of the compounds in soap made from most oils, but it's particularly prevalent in cocoa butter, pork lard, illipe, and shea nut soap.
The exact proportions of oil to lye different from oil to oil, and are detailed in the recipe section of this site.